Obesity, a dangerous condition that can progress to hypertension, diabetes, systemic inflammation, and cardiovascular disease, has long been researched by scientists. Obesity and cancer have also been linked in studies; the latest data suggest that smoking, alcohol use, as well as obesity are the leading causes of cancer globally, as per WHO.
Fat cell formation, which begins with a small fibroblast-like progenitor, not just activates the fat cells' particular genes but also causes them to enlarge by accumulating additional lipids (adipose tissue and adipocytes). In reality, lipid storage is a fat cell's defining role. However, excessive lipid accumulation can cause fat cells to be dysfunctional and contribute to obesity.
Many people's prayers would be answered as scientists could pinpoint fat cells and securely decouple harmful fat accumulation from good fat metabolism. A significant obstacle in the treatment of obesity is that subcutaneous fat, which is not continuously throughout the system but is detected piece by piece in "depots," has proven to be difficult to address in a depot-specific, precise manner.
There have been two types of fat: visceral fat, which surrounds the abdomen, and liver, including the intestines, plus subcutaneous fat, which is found underneath the skin everywhere in the body. Visceral fat causes potbellies; subcutaneous fat causes chin jowls, arm fat, and so on. There has never been a mechanism to selectively cure visceral adipose tissue. And existing therapies for subcutaneous fat, such as liposuction, are intrusive and harmful.
Cationic Nanomaterials Targeting Fat Cells
Two new experiments from Columbia Engineering as well as Columbia University Irving Medical Center (CUIMC) researchers may hold the key to targeting fat cells depot-specifically and safely. The articles present a novel strategy for treating obesity that involves the use of cationic nanomaterials which can target particular locations of fat and prevent the harmful buildup of larger fat cells. The materials modify fat instead of destroying it as liposuction does.
The initial study, published in Nature Nanotechnology, emphasizes targeting visceral adiposity or abdominal fat. The second research, which was published online by Biomaterials on November 28th, focuses on fat within the skin in addition to systemic inflammation linked with obesity.
The research team, led by Li Qiang, assistant professor of pathology and cell biology at CUIMC, as well as Kam Leong, and Samuel Y. Sheng Professor of Biomedical Engineering and systems biology at CUIMC, discovered that adipose tissue contains a large amount of negatively charged extracellular matrix (ECM) to hold fat cells. They reasoned that such a negatively charged ECM network may act as a type of freeway system for positively charged particles.
So they treated obese mice with a positive charge nanomaterial has known as PAMAM generation 3 (P-G3). The P-G3 propagated fast throughout the tissue, and the researchers were ecstatic that their strategy of particularly targeting visceral fat had worked. Then something strange happened: P-G3 turned off lipid storage management in fat cells, causing the mice to lose weight. This was completely unexpected, considering P-well-established G3's role in disarming negatively charged organisms, including such DNA/RNA cell debris, to reduce inflammation.
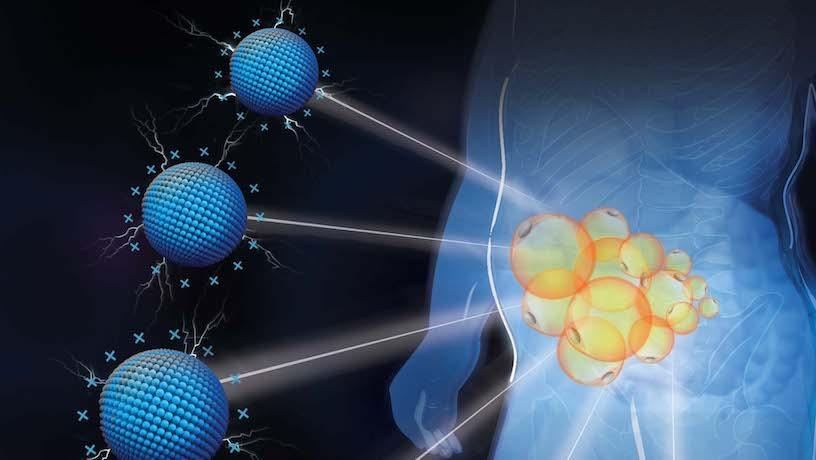
Illustration of depot-specific targeting of fat by cationic nanomaterials. Columbia researchers discover that the cationic charged P-G3 reduces fat at targeted locations by inhibiting the unhealthy lipid storage of enlarged fat cells
ALSO READ: Obesity Diagnosis: Study Shows New Formula for Health Measurement
P-G3: Cationic Nanomaterial Substance
The researchers noticed in these two tests that the cationic substance, P-G3, could do an interesting thing to fat cells: although it assisted new fat cell development, also it uncoupled lipid storage of fat cell housekeeping tasks. Because it prevents larger fat cells from storing harmful lipids, the mice had much more metabolically healthy, youthful, tiny fat cells like those seen in newborns and athletes, as described by the university paper.
The researchers discovered that this uncoupling activity of P-G3 is also found in human fat samples, indicating the possibility of translating in humans. Fat cells can remain fat cells with P-G3, but they can't grow up, according to Leong, a pioneer in employing polycation to scavenge diseases. These findings highlight an unexpected technique for treating visceral obesity and point to a new path for investigating cationic nanomaterials for the treatment of metabolic disorders.
Leong and Qiang see various possibilities for their ability to specifically target visceral fat. The Biomaterials study reveals a straightforward technique that might be employed for cosmetic objectives; similar to Botox, P-G3 can be injected locally into a particular subcutaneous fat depot.
The scientists, who have patents filed, are currently modifying P-G3 to increase its efficacy, safety, as well as depot specificity. Just what researchers are most enthusiastic about is transforming P-G3 into a system that can deliver medications and gene treatments to specific fat depots. Thiazolidinediones (TZDs), an effective but risky medicine that is a strong regulator of fat and is employed to cure type 2 diabetes-but it is related to heart failure and prohibited in numerous countries-could be repurposed as a result of this.
Qiang expressed his excitement at discovering that cationic charge is the key to targeting fat tissue. Humans can now shrink fat depot specifically-anywhere they want-and safely without damaging fat cells. This is a significant step forward in the treatment of obesity.
RELATED ARTICLE: Can Obesity Be Linked to ADHD? Here's What Science Says
Check out more news and information on Obesity in Science Times.