The US Centers for Disease Control and Prevention approved the agency's Vaccination Advisory Committee's decision to pave the path for Pfizer and BioNTech vaccines to be distributed in the US.
The news accompanied the decision by the Advisory Committee on Immunization Policies (ACIP) of the CDC on Saturday to prescribe the vaccine to citizens 16 and over. That emerged after the vaccine was approved for immediate use on Friday by the US Food and Drug Administration.
Here's what we learned about the side effects of the COVID-19 vaccine from Pfizer/BioNTech.
Adverse Effects
More adverse effects were seen from people under 55 years of age than by older volunteers, as reported by Business Insider. These side effects were suspected for a vaccination, usually arising within a few days of delivery of a dosage and continuing on average for just a day or two.
Usual side effects include:
- Fever (14%)
- Joint pain (24%)
- Chills (32%)
- Muscle pain (38%)
- Headache (55%)
- Fatigue (63%)
- Pain at the vaccination site (84%)
Information about the dangerous side effects that volunteers suffered was also given by Pfizer and the FDA. There are in general, side effects that are so severe that they prohibit persons from undertaking daily activities.
Much of the significant side effects emerged after the second dose was given to the patients, and these side effects were more prevalent in the younger participants.
Extreme Fatigue and Headaches
For individuals aged 18 to 55, after having their second dose, 4.6 percent recorded extreme exhaustion, and 3.2 percent experienced severe headaches.
Fevers were normal in that age group as well during their second shot, around 15.8 percent of the volunteers had a fever of at least 100.4 degrees Fahrenheit.
2.8 percent recorded extreme exhaustion in volunteers older than 55, 0.5 percent had severe headaches during the second dose, and 10.9 percent had a fever.
To see whether there were any unforeseen safety issues, researchers at the FDA and at Pfizer and BioNTech are now sifting through the results. Encouragingly, they said that they did not see something that triggered worry in documents published Tuesday.
FDA regulators noticed a "numerical imbalance" in cases of Bell's palsy, a disorder that momentarily weakens facial muscles: There were four cases of Bell's palsy in the 20,000-plus people who received Pfizer's vaccine, contrasting with no reports in the vaccination community.
But the FDA said that for those who received Pfizer's dose, the incidence of Bell's palsy cases was not much greater than what might be predicted for the general public.
Pfizer/BioNTech COVID-19 Vaccine Still Good?
Despite the side effects, the FDA said evidence showed that the mRNA vaccine course of Pfizer performed well in thousands of individuals who studied it in six countries of diverse ages, ethnicities, and genders.
One graph in the briefing materials reported how many individuals received COVID-19, the pathogen triggered by the novel coronavirus, during the analysis. It reveals exactly how good the two-shot vaccination course performed to avoid infections by contrasting people that received the Pfizer vaccine with those in the control group.
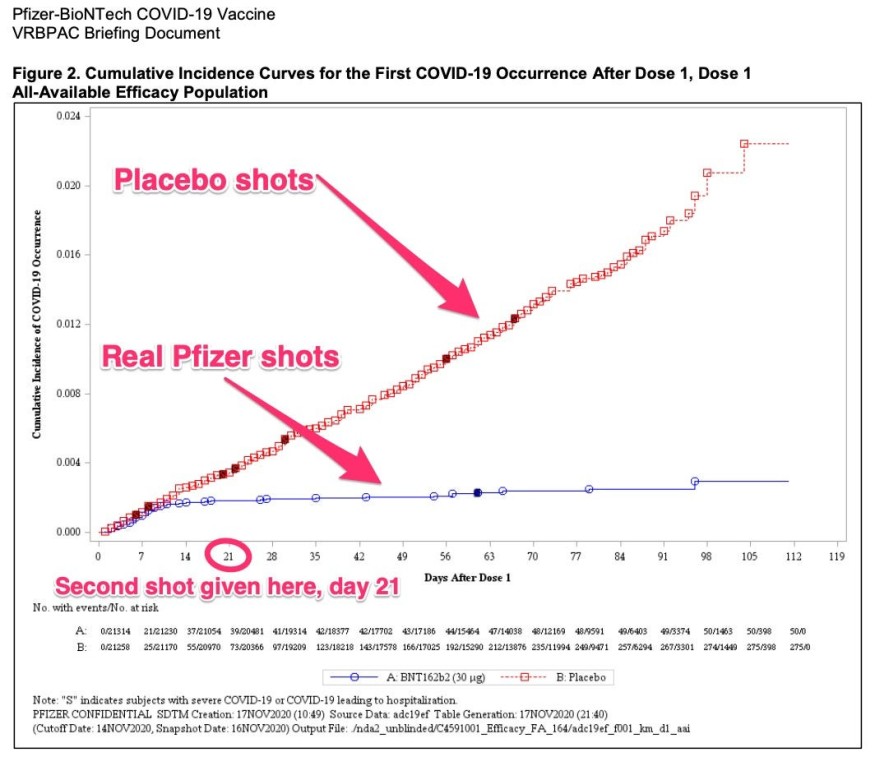
The red line in the map indicates how many of the control group's nearly 21,000 people, all who (unknowingly) received a false (placebo) hit, got ill. Of the nearly 21,000 individuals who got the actual Pfizer injection, the blue line indicates the COVID-19 infection rate.
The chart shows that 14 days after their first injection, people who had the actual Pfizer vaccine continued to receive immunity against infection. Then, they received their second and final) chance 21 days later. After that for at least two more months, they were well covered from infection.
The safety of the vaccine may last even longer than that. Only it's too premature to tell for sure.
Improved Vaccine Safety
For shots to take place in the body, it requires some time to have a stronger and complete defense against the virus. After getting the first Pfizer injection, some became ill with COVID-19 before receiving their second shot.
Yet beginning one week after the second injection, the vaccination was even more effective at avoiding infections. The 2-shot course had a 95% effectiveness of the vaccine.
Since getting both doses of the Pfizer vaccine and awaiting a full week for the injection to come into effect, only eight individuals obtained COVID-19 (roughly 28 days from the start of the trial). Among those eight, only one person experienced a significant case of the disease but they were not hospitalized.
Check out more news and information on COVID-19 on Science Times.












