After a little over a year of expedited research, financial hardship and over 500K deaths in the US, we're finally starting to see the light at the end of the COVID-19 tunnel. But while the widespread rollout of vaccines has been encouraging, there are many additional steps we will have to take for the next few months, possibly years, to stop the spread of the virus.
According to Pharmaceutical Technology, more than 57 million people have been fully vaccinated worldwide. Many people are looking at a person's vaccination status as a type of "passport" to measure whether they are safe to travel and see friends/family but it does not actually reveal someone's true levels of immunity at any given time. Recent research shows varying speeds of neutralizing antibody drop-off, but a few specific studies have shown that levels start to fall about 60 days after receiving the second dose of the vaccination. If a person was vaccinated say six months before they travel, are they still immune to the virus?
So what's next?
Instead of a "vaccine passport", vaccinated individuals should start to look for an "immunity passport" to determine whether they are actually safe and immune.
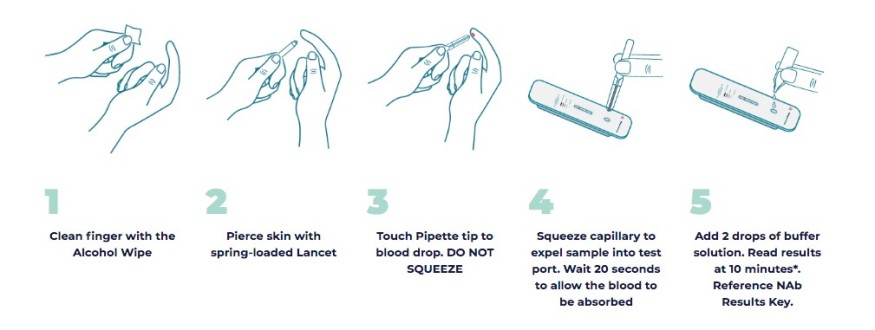
AXIM Biotechnologies has created a rapid point-of-care test called ImmunoPass that, within 10 minutes, can measure a person's levels of neutralizing antibodies to see if they are high enough to fight off the COVID-19 virus, especially while traveling. Preliminary studies are also providing evidence that people who exhibit high levels of neutralizing antibodies may be less likely to pass on the virus to others, decreasing the overall risk of onboard transmission.
ImmunoPass, which AXIM and its manufacturing partner Empowered Diagnostics are in the process of submitting a new application for FDA Emergency Use Approval for, has already been proven to work accurately in plasma and serum with 97.8% accuracy. With this next round of human clinical trials, AXIM aims to show that not only can they use plasma and serum to get results, but also whole blood -- allowing for patients to conduct the test with a single drop of blood via a finger prick.
A priority for this type of rapid neutralizing antibody test is to make it easily accessible so AXIM hopes to provide this test to pharmacies so that consumers can conduct the test safely at home and send the results to their doctor afterward.
This will also prove helpful when measuring if a vaccinated person needs a booster dose of the COVID-19 vaccine they received. As new variants emerge around the world and the FDA works to create guidelines for vaccine manufacturers to market additional booster shots, this type of testing will help them recognize trends in neutralizing antibodies levels based upon location, age and other demographic factors.
While "Vaccine Passports" seem much easier for the general public to obtain and understand, "Immunity Passports" are the only way that we're going to be able to safely travel, see our loved ones, and go back to any sense of normal in months and years to come.
© 2025 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.