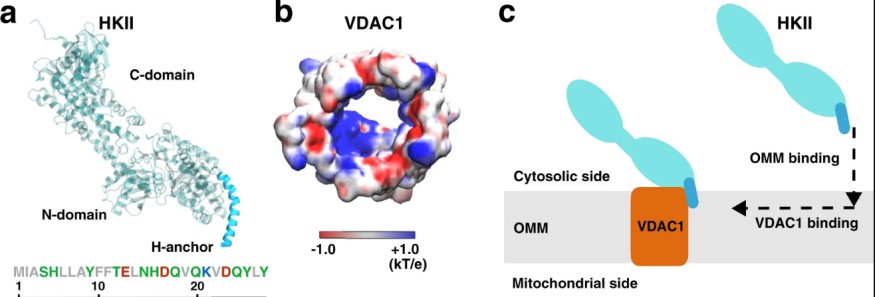
Supercomputer simulations are recently developed by experts to understand more about the correlations between a mitochondrial-assisting cell and a glucose metabolism regulator. The cellular bodies are known as the cell mitochondrial voltage-dependent anion channels or VDACs, and the enzyme hexokinase-II or HKII. Among the discovery was the binding activity between the two cellular bodies, where the cytosolic enzyme hexokinase is attached to the surface of a mitochondrial membrane and is supported by the endogenous membrane protein. Across the vicinity of the VDAC and the mitochondrial surface is the attachment of HKII. The new study is expected to help scientists dig deeper into the molecular properties that relate to the deadliest disease: cancer.
Cellular Binding and How Cells Transfer Energy Shown in Supercomputer Simulation
The simulations developed are beneficial for the molecular and cellular scientists on their venture to understand how specific cells work. In addition, the simulation can also produce other observations gathered when intracellular proteins react to other types and how cells give off signals to complement or interact with other variants for regulation and other cell functions.
Adenosine triphosphate or ATP transfers energy from various cellular bodies. It acts as the currency that fuels the functionality of every cell. According to Florida News Times, among the key interests of the study is the potentials produced whenever ATP interacts with the power-consuming cells such as the protein enzyme hexokinase-II and the voltage-dependent anion channel, which are observable just right outside the surface of the mitochondrial membrane.
Supercomputer simulations utilized in the study are among the first to show how the VDAC binds with HKII. Because of the simulation and the detailed analysis that could be gathered from the model, the study by the Texas Advanced Computing Center or TACC was awarded Extreme Science Engineering Discovery Environment or XSEDE by the National Science Foundation.
ALSO READ : Fossilized Cretaceous Bird With Fancy Tail Feathers First to Express Evolutionary 'Fashion Over Function'
Cellular Activities Outside the Mitochondrial Channel
The basic aim of the research is to know how these proteins interact just outside the cell powerhouse, which is theorized to hold the key to answering important questions surrounding cancer. The University of Illinois at Urbana-Champaign biochemistry expert and co-author of the study Emad Tajkhorshid said they already got ahold of the evidence regarding how the two proteins bind, but the process involved in the binding phase is what inspired the team to conduct the simulation.
Stampede2 was the technology used for identifying the binding process of the proteins. In the study, it was found that the conduction of energy in between the cellular channels shifts and eventually blocks the ATP from cell individuals as the binding phase of the enzyme and proteins attaches. These findings are located in the advanced, off-site XSEDE storage architecture of TACC called the Ranch system.
The XSEDE collaboration helped the TACC project significantly in studying the complexities of a biological system involving the mitochondria and other proteins. The simulations have put their studies one step ahead, as observation of the cellular bodies conducting their activities in real-time is challenging for biology experts. The study was published in the journal Communications Biology, titled "Structural basis of complex formation between mitochondrial anion channel VDAC1 and Hexokinase-II."
RELATED ARTICLE : Phytoplasma Bacteria Knows How to Parasitize Plants; Slows Age Development and Turn Into 'Zombies'
Check out more news and information on Biology in Science Times.











