The COVID-19 antiviral drug Paxlovid is intended for those who will likely become seriously sick from the virus, like older people with pre-existing conditions. The US Food and Drug Administration (FDA) first approved its use in December. But two weeks ago, FDA authorized pharmacists to prescribe the antiviral drug.
It comes after months of discussion to increase access to the drug based on studies that showed a decrease in mortality rate and hospitalization in unvaccinated patients by almost 90%. However, the drug was found to be less effective in vaccinated patients.
US President Taking Paxlovvid to Fight Off COVID-19 Infection
Last month, US top infectious expert Dr. Anthony Fauci announced that he took a second course of Paxlovid after testing positive again with COVID-19. Then on July 21, the White House announced that US President Joe Biden is taking Paxlovid against COVID-19 after he was diagnosed with the deadly disease.
"He has begun taking Paxlovid. Consistent with CDC guidelines, he will isolate at the White House and will continue to carry out all of his duties fully during that time," Press Secretary Karine Jean-Pierre wrote in the announcement.
The president's physician Dr. Kevin O'Connell said that Biden's symptoms began on Wednesday evening. Since he had a history of heart condition atrial fibrillation since 2003, he was advised to take Paxlovid. However, there are concerns that it could negatively react to his medications, such as his blood thinners to treat the heart condition.
Last fall, Dr. O'Connell said that the president remains asymptomatic of atrial fibrillation but did not mention any medications Biden is taking to mitigate it. Aside from his heart condition, the president also has asthma, which does not have any adverse risks associated with the COVID-19 antiviral drug but can increase his risk of COVID-19 complications.
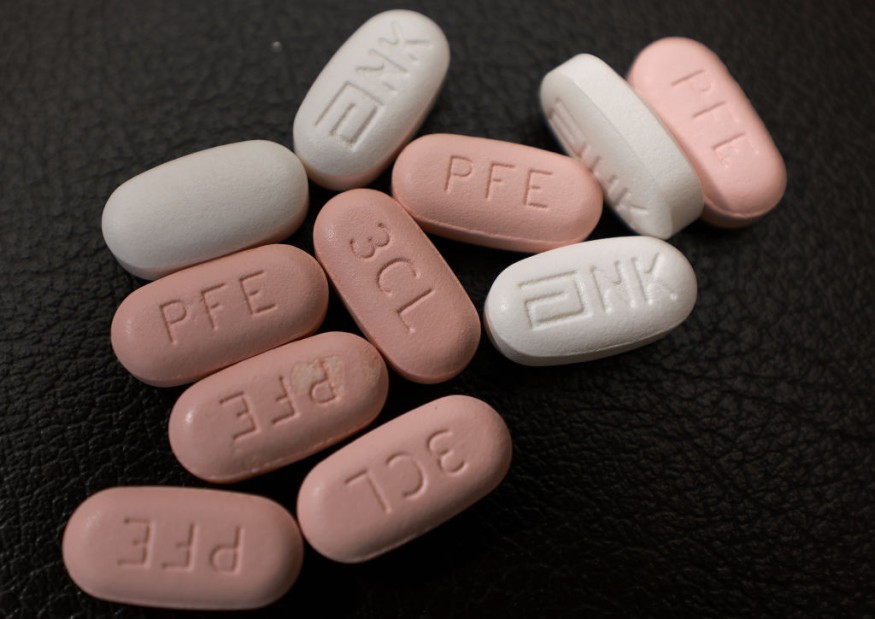
According to MailOnline, the treatment course off Paxlovid consists of a three-pill dose of two different types of medications in which each set of pills should be taken twice a day for five days until all 30 pills are consumed for it to be effective.
Two of the pills in one dose inhibit the enzyme that allows SARS-CoV-2 to replicate to stop it from spreading to other healthy cells, while the third pill is a medication previously used to fight HIV/AIDS and boosts antiviral defenses by shutting down the metabolism of the liver to allow the effects of the first two pills to remain longer in the body.
Side Effects of Taking Paxlovid
According to Yale Medicine, Paxlovid does not typically cause any side effects, although there are cases when it can cause increased blood pressure, nausea, diarrhea, and muscle aches. The Pfizer-produced antiviral drug is not recommended for people with liver or kidney disease and even for those with atrial fibrillation like Biden.
Pfizer noted that studies showing side effects of Paxlovid report mild side effects that affected relatively few people, especially since not many have taken the drug. However, they think that severe and unexpected side effects may still happen and that they are continuously studying Paxlovid to identify all unknown risks.
The Atlantic previously reported that a clinical trial by Pfizer showed that 5.6% of the participants reported having dysgeusia, a disorder that alters the sense of taste to make foods taste bitter or metallic. But these cases were only mild and a few discontinued the use of the drug.
RELATED ARTICLE: Dysgeusia a Common Side Effect Among Recipients of Coronavirus Antiviral Drug Paxlovid From Pfizer [REPORT]
Check out more news and information on COVID-19 in Science Times.
© 2025 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












