Tags: COVID-19 vaccine

Pfizer-BioNTech Vaccine Likely To Work Against New COVID-19 Strain In UK
New COVID-19 Strain Discovered in L.A., but Researchers Say It Was First Identified in Denmark
Experts Say Allergies to COVID-19 Vaccine May Happen; What Does That Mean?
Moderna COVID-19 Vaccine: Here's What You Need to Know

COVID-19 Vaccine's Second Dose May Cause Side Effects

COVID-19 Vaccine Working at Room Temperature And Requires Single Shot, In The Works

Severe Allergic Reactions to COVID-19 Vaccines Are Highly Unlikely
COVID-19 Vaccines: What Data Can Tell Us About Allergic Reactions and Anaphylaxis

COVID-19 Vaccine Potentially Ineffective Against New South African Variant
Use of COVID-19 Vaccine Developed in India Alarms Scientists for Lack of Trials

South African Scientists Strive to Understand New COVID-19 Variant

China State Developed Vaccine Administration Begins, 79% Effectiveness Claimed; World Scientists Seeking Details

Oxford-AstraZeneca Vaccine Authorized in UK, 100M Viles Ordered
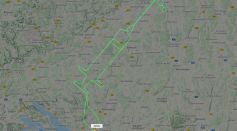
COVID-19 Vaccine Celebrated by a Pilot Plotting a Giant Syringe in Sky
Here's What Polio Vaccine Developer Thinks About COVID-19

COVID-19 Vaccine: A 101-Year-Old Woman First Received COVID-19 Vaccine in Germany

New COVID-19 Mutated Strain More Contagious: Will the Coronavirus Vaccine Work On It?

Pfizer Has Made Public the Full Ingredients of Their Vaccine
FDA Advisory Committee Recommends Second COVID-19 Vaccine For Emergency Use Authorization
Absence of COVID-19 Vaccine Delivery Plan Could Shake Public Trust
Most Popular

Memory and Learning: How the Brain Stores, Retrieves, and Forgets Information

Gut Microbiome 101: How Gut Bacteria Influence Immunity, Mood, and Metabolism

The Strongest Tornadoes Ever Recorded: Scientific Breakdown of Extreme Tornado Events

How Solar Activity Shapes Our Planet: What the Next Solar Maximum Means for Earth





