vaccines
COVID-19 Vaccines May Be Administered Through the Nose Soon
Chinese Vaccines "Don't Have Very High Protection Rates," Says China CDC Official
What Causes Blood Clots on AstraZeneca COVID-19 Vaccine?
Pfizer Seeks FDA’s Approval for Younger Population’s Vaccination Aged 12 to 15 Year Olds
Moderna Vaccine Side Effect Includes "Covid Arm" or Rashes on the Skin; Should We Worry?
AstraZeneca COVID-19 Vaccine Efficacy: Stunning Images Reveal How Injected Person’s Own Cells Replicate Virus Spike to Combat Infection
Just Got the First Dose of COVID-19 Vaccine? How Important is the Second Shot?

5 Best Foods to Eat Before, After Setting Appointment for Your COVID-19 Vaccine
Adolescents Show High Tolerance on Pfizer-BioNTech COVID-19 Vaccine, 100-Percent Efficacy
Pfizer-BioNtech COVID-19 Vaccine Can Now Be Stored at Higher Temperatures

Pfizer-BioNTech Launch Trials to Test COVID-19 Vaccine on Kids as Young as Six Months
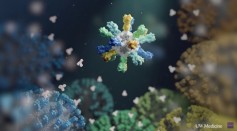
Nanoparticle Flu Vaccine Found Effective in Animal Trial, Blocks Seasonal and Pandemic Strains Like H5N1

Can Vaccinated People Get Infected With COVID-19?
AstraZeneca COVID-19 Vaccine: Safety and Efficacy Revealed in Large Clinical Trial Results
Most Popular

China’s Tiangong Space Station to Expand Its Capabilities With New Modules
AI Revolution in Medical Education: Transforming How Healthcare Professionals Learn

Exploring Life Beyond Earth: Study Claims Other Planets Could Be Suitable for Alien Life

Out of Office, Not Out of Mind: Planning for Employee Holiday Absences