
The reality—our increasingly polluted environments call for increasingly efficient technology that will help both scientists and consumers in their daily activities. However, considering the analysis of pollutants in the air, current techniques involve gas chromatography–mass spectrometry, or GC–MS, which takes a bit of time although efficient. And in cases where a seemingly instant result is needed or where contamination must be at a minimum, air samples cannot wait a few hours or a few days for GC–MS to do its job.
With this in mind, researchers from Nanyang Technological University, Singapore, have recently published a report in ACS Nano, where they describe a system they have developed to improve air analysis. The device, as they explain, can provide real-time analysis of pollutants in the air.
The researchers looked into Raman spectroscopy, a technique that has been in use in the analysis of solid and liquid samples. However, because of the less organized molecular structure of gaseous samples, analytes appear to be too dilute to be analyzed using this technique. Considering this restriction, NTU Singapore associate professor Ling Xing Yi and PhD student at the university Phan Quang Gia Chuong came up with a nanostructure using a highly porous metal-organic framework, or MOF.
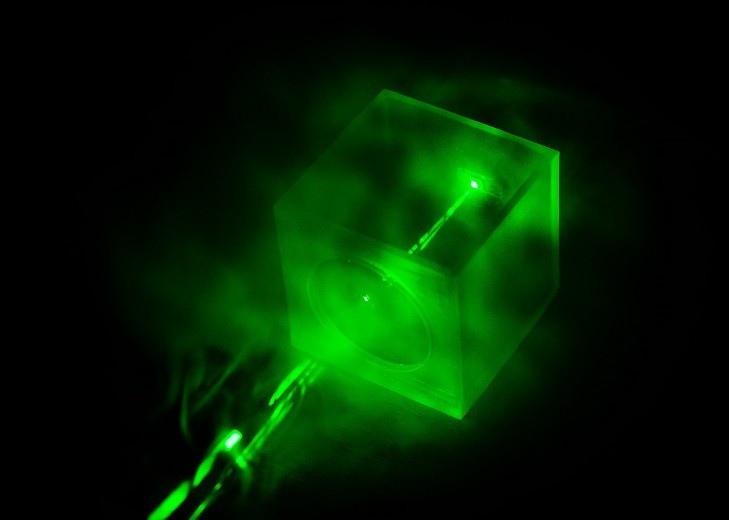
The device is made of a porous metallic nanomaterial that traps gas molecules. Light from a laser then comes in contact with the molecules, resulting in the emission of low-energy light. After this, a spectroscopic reading will be available for the user in the form of a graph. This graph, which is unique for each analyte, acts as a chemical fingerprint. Referencing a library of known chemical fingerprints will help identify the chemical pollutant present.
The use of a nanostructure composed of metal nanoparticles also results in a sort of upgrade—a "millionfold enhancement"—from the current Raman spectroscopy framework. The researchers claim that the identification of trapped molecules in the structure can be attributed to the metal nanoparticles that intensify the light surrounding the molecules.
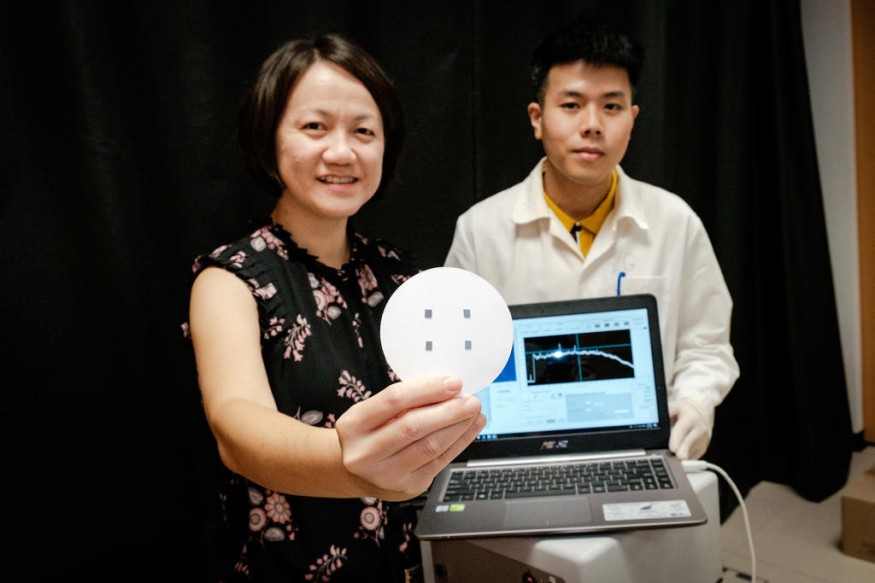
The researchers have conducted experiments where the device was able to identify polyaromatic hydrocarbons, which are airborne molecules and were identified in the past as carcinogenic. Examples of these are naphthalene and benzene, chemicals often used in the petrochemical industry.
Of course, the researchers also had to consider the safety of the users. They looked into how effective the device is when used from a distance in order to minimize hazards. In tests, the device was able to work up to a distance of 10 meters or 32.8 feet. For future work, the laser can be engineered to reach greater distances.
The researchers are already working on the technology patent and its commercialization.











