Experts from the US health regulatory agencies have recently announced plans to conduct a clinical trial for a monkeypox antiviral drug to deal with the rapidly rising cases in the country.
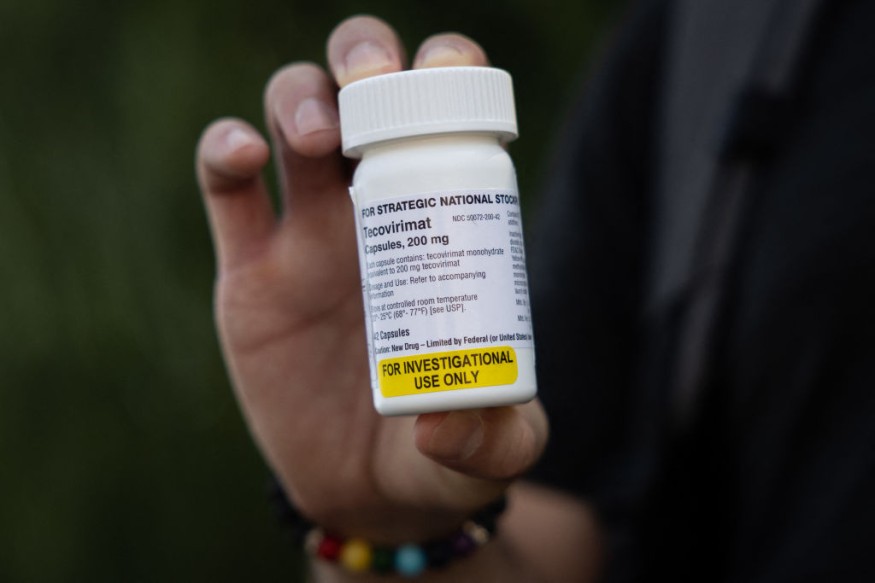
The drug is called Tecovirimat, sold under the name TPOXX from the New-York-based pharmaceutical company SIGA Technologies, Inc. and is approved for use in humans to treat smallpox. The clinical trial aims to thoroughly assess the safety and efficacy of an antiviral drug in monkeypox patients.
US FDA Has Yet to Give TPOXX the Greenlight
The US Food and Drug Administration (FDA) so far only allows JYNNEOS Vaccine to prevent smallpox and monkeypox in adults 18 years old and above at high risk of infection. So far, the immunological response to the vaccine administration has consistently prevented the disease.
However, there is no FDA-approved or authorized medicine for monkeypox treatment yet. The TPOXX antiviral medication is only available through the Centers for Disease Control and Prevention (CDC) under the Expanded Access or "compassionate use" authority given by the FDA.
As a result, very few patients have received the antiviral drug and both patients and doctors must go through a stringent and cumbersome process before getting the antiviral drug.
The two health agencies continue to work together to streamline their Expanded Access Program for the viral disease to improve access to medication. But there are no current human data demonstrating the effectiveness of Tecovirimat as a treatment of monkeypox disease in humans.
The global monkeypox outbreak has already caused about 5,800 probable and confirmed cases in the country. The US FDA has posted more information about TPOXX's approval for smallpox under the administration's "Animal Rule" on its monkeypox webpage.
Clinical Trial Needed for TPOXX
CNN reported that TPOXX has been administered to over 223 monkeypox patients since July 22, which is way below the confirmed or probable infections in the country that has already reached more than 6,600 as of writing.
An approval from the FDA would give easy access to patients and supply would not be an issue either, as the US Strategic National Stockpile already had 1.7 million available courses of the antiviral drug even before the outbreak happened.
But before that, health regulatory experts from the National Institute of Allergy & Infectious Diseases, CDC, and FDA wrote in The New England Journal of Medicine that a clinical trial is still needed. They noted that human trials for monkeypox are not only ethical but also feasible and for the best instead of basing its safety and efficacy data on animal trials alone.
For now, the details of the clinical trial have remained hazy and SIGA CEO Philip Gomez told BioSpace that they are currently working closely with NIAID. They anticipated the trial to start soon. He added that they are also working with various governments to secure stockpiles of TPOXX ahead of its regulatory clearance.
That means patients have little choice other than to wait for clinical trials to wait or try to access the drug under the Expanded Access program of the FDA.
RELATED ARTICLE: New York City Opens Pop-up Mass Vaccination Sites to Provide Monkeypox Vaccine to Residents
Check out more news and information on Monkeypox in Science Times.











