moderna
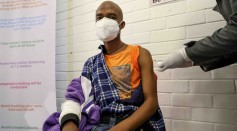
COVID-19 Vaccines May Damage Human DNA

Moderna Announced Partnership With Amazon Web Services for Their Analytics and Machine Learning Services

Is Moderna Coronavirus Vaccine Leading the Race? Early Trials Show the Jab Gives Off Immunity
Volunteer Suffered 'Severe Reaction' in Moderna Therapeutics' Coronavirus Vaccine Trial

Moderna’s COVID-19 Vaccine Dubious? Experts Demand Concrete Data Not Mere Words
'Very Low' Dose Moderna COVID-19 Vaccine Elicits Immune Response With No Side Effect, First Human Trial Show

Production of the First Batch of Proposed COVID-19 Vaccines in the US Expected in July
Most Popular

November’s Beaver Moon: Final Supermoon of the Year Peaking Mid-Month
Cloud Adoption and Migration Best Practices Key Points to Include

HIPAA Compliance in the Age of Big Data

Rare Fossil Reveals Key Clues About Evolution of Bird Brains from Dinosaurs






