Rice University engineers use copper cubes as a catalyst to the new reactor to turn carbon monoxide into acetic acid. According to New Atlas, copper cubes as catalysts make the new reactor relatively simple to operate and allow it to last for long periods to turn unwanted waste gas into an industrially useful product.
Carbon monoxide is a by-product of industrial processes that can be indoor or urban air harmful to human health. However, it does not directly become a greenhouse gas in the atmosphere. On the other hand, acetic acid is the tang in vinegar and is often used in foods, antiseptic, solvent, and various industrial processes and products.
Therefore, converting the harmful waste into something industrially useful is practical. They published their study in the Proceedings of the National Academy of Sciences.
Nanosize Copper Tubes As Catalyst to the New Reactor
Biomolecular engineers Haotian Wang and Thomas Senftle of Rice's Brown School of Engineering took inspiration from a previous device used to convert carbon dioxide into formic acid. In their laboratories, the electrochemical process helped resolve issues in that device, which required additional steps to make the device environment friendly.
"We're upgrading the product from a one-carbon chemical, the formic acid, to two-carbon, which is more challenging," Wang said. "People traditionally produce acetic acid in liquid electrolytes, but they still have the issue of low performance as well as separating the product from the electrolyte."
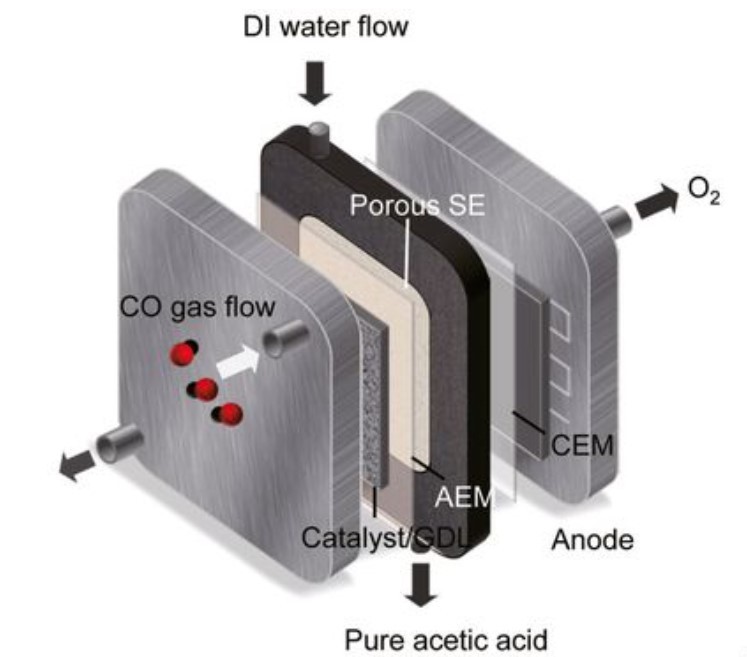
The environment-friendly device now uses nanoscale copper cubes and a unique solid-state electrolyte as a catalyst. It produced 2% acetic acid in water after 150 hours of continuous lab operation, Science Daily reported.
Wang added that there are times when the copper cubes would produce chemicals other than acetic acids, such as alcohols. As a solution, they engineered the copper cubes to only one facet, which could help the carbon-carbon coupling direct it to produce only acetic acid and not other products.
Then as they add deionized water to the reactor, it mixes with acetic acid and creates a usable solution. New Atlas reported that the remaining gas in the process is released as oxygen.
A Good Demonstration of How Theory and Experiment Interacts
According to Senftle their project is a good demonstration of how well theory ad experimentation should mesh because it exemplifies how the many levels of engineering could fit the themes of molecular nanotechnology to show how it can be scaled up to real-world devices. The researchers were able to integrate components of a reactor all the way down to the mechanism at the atomistic level.
In the next step of making a scalable system, they plan to improve the stability of the system and further reduce the amount of energy need for the process, according to Wang.
Check out more news and information on Nanotechnology on Science Times.












